This answer has been confirmed as correct. The root of the anion name is listed second and is followed by the suffix idean anion is a negative ion.

Naming And Writing Formulas Of Binary Ionic Compounds Chemistry Lesson Youtube
Binary molecular compounds that is covalent compounds that contain only two nonmetal elementsare named with a system utilizing Greek prefixes to indicate the number of atoms of each kind.

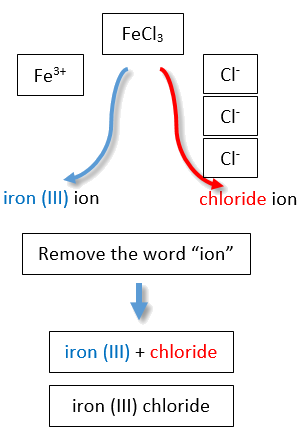
. Naming NaCl is a simple process. Binary ionic compounds are named in what pattern. Binary ionic compounds are named by writing the name of the action followed by the name of the anion.
For binary ionic compounds ionic compounds that contain only two types of elements the compounds are named by writing the name of the cation first followed by the name of the anion. What is the pattern of an ionic compound. The procedure diagrammed in Figure 43 1 uses the following steps.
Na 3N sodium _____ 2. The metal followed by nonmetal. Binary ionic compounds are named in the following pattern.
If the anion is bromine the name will be bromide and so on. Binary ionic compounds are named by metal followed by nonmetal. The nonmetal followed by the metal.
It is used to preserve season and flavor foods and can be found in saltwater. Log in for more information. There can be one of each element such as in sodium bromide or potassium iodide.
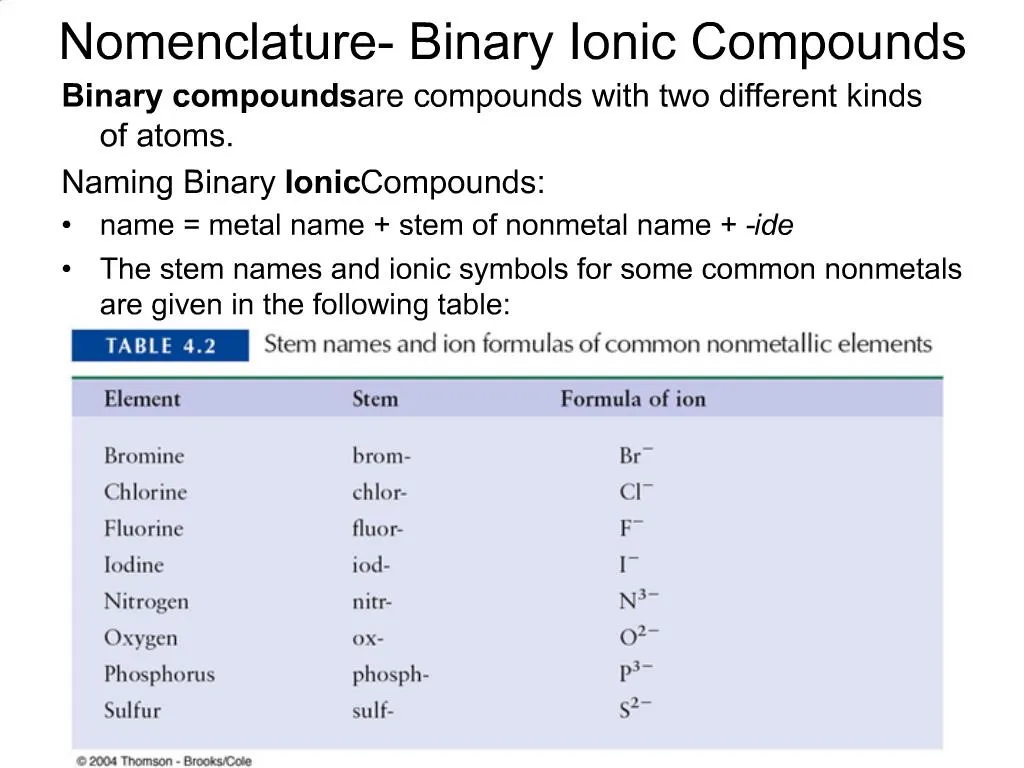
One example is MgCl 2 a coagulant used in the preparation of tofu from soybeans. Na Sodium Mg 2 Magnesium Al 3 Aluminum. The anion is named after using the suffix -ide.
What pattern are binary ionic compounds named in. The metal followed by the polyatomic ion. Keys to Naming Binary Ionic Compounds.
Binary ionic compounds are named in what pattern. Added 1222019 93921 PM. Figure 43 1 Naming a Covalent Inorganic Compound.
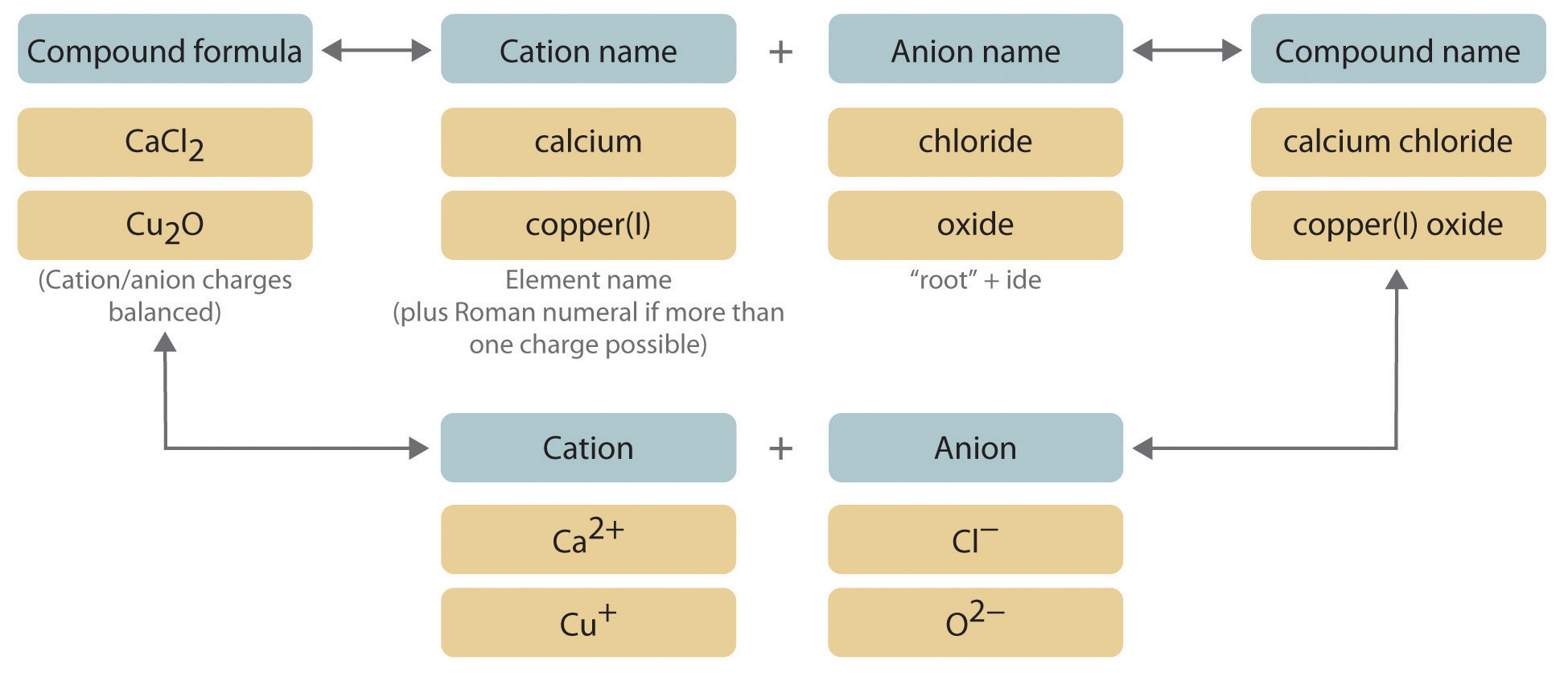
CaCl 2 Calcium chlor ine Calcium chlor ide. Ionic compounds form large repeating structure of ions. This structure is known as a crystal lattice.
KBr potassium _____ 3. Naming Ionic Compounds from saylordotorggithubio Binary molecular compounds binary molecular compounds that is covalent compounds that contain only two nonmetal elementsare named with a system utilizing greek prefixes to. Binary ionic compounds are named by metal followed by nonmetal.
NaCl is a very common binary ionic compound. Rules for the naming of Binary Ionic compound. If the metal atom is Transition element then the roman numeral is included after the metal name to indicate the oxidation number of the metal.
Want this question answered. First write the full name of cation. Formulas of Binary Ionic Compounds.
Metal followed by the nonmetal. For the non-metal the anion write the name on the Periodic Table and then replace the ending with ide. An ionic compound that contains only two elements one present as a cation and one as an anion is called a binary ionic compound An ionic compound that contains only two elements one present as a cation and one as an anion.
Examples of Naming Binary Ionic Compounds. Complete the names of the following binary compounds. Be notified when an answer is posted.
Al 2O3 aluminum _____ 4. The anion followed by the cation. Binary ionic compounds are named in what pattern A The metal followed by the from SCIENCE 021 at James Madison High School.
Log in for more information. Potassium bromide is an example of an ionic compound. MgS _____ Formulas for Binary Ionic Compounds A binary compound is one made of two different elements.
Name the metal the cation as it appears on the Periodic Table.
5 7 Naming Ionic Compounds Chemistry Libretexts

Forming And Naming Ionic Compounds Type 1 And 2 Binary Compounds Ppt Download

Solved That They Do Part I Nomenclature Of Binary Ionic Chegg Com

Solved Part Ii Binary Ionic Compounds Containing Chegg Com

Naming Ionic Compounds A Guided Inquiry Exercise
Ppt Nomenclature Binary Ionic Compounds Powerpoint Presentation Free Download Id 721132

0 comments
Post a Comment